by Kendall Smith, Induron Power Market Manager
You know you need a really great and unique coating. The coating has to be applied by guys who have climbed to the top of your high-voltage electric transmission towers, which may even be energized, and perform surface preparation and coatings application while climbing the structure (very high degree of difficulty and dangerous).
Since SSPC-SP2 Hand Tool Cleaning is often the only practical and cost-effective method of surface preparation, your coating must have excellent surface wetting and penetration of rusted substrates and good adhesion to galvanized steel, as well as rusty carbon steel.
Since each climb is very costly, the coating must also have high-build properties (to obtain heavy DFTs in one coat), and be easy to apply by a climbing painter, with mitts, brushes, or rollers. When the coating has been applied and cured, it must protect the substrate from corrosion by building an effective shield or barrier against all types of environments, including extreme UV, heavy rain, industrial and marine exposures. The more carefully you investigate what types of coatings have worked best in the past, the better you will write your specification today! It is safe to say that it has never been about one ingredient alone.
Post-WWII, red lead in various drying oils were used as primers. Aluminum pigmented oils were used as finish coats, and two to three coats were required. A three-coat system might last 12 years in a moderate exposure, and only 5-8 years in an aggressive exposure. A significant improvement came in the late 1950s by combining the lead pigments with aluminum and zinc dust, and also increasing the solids-by-volume, from 50+% to 90+% SBV. This allowed for a much higher WFT and DFT as compared to earlier coatings. Also note that with leafing aluminum, the formulator was combining a “labyrinth effect” pigment in combination with zinc dust; plus a corrosion inhibitor, making a difficult pathway for moisture and corrosives to penetrate the film down to the substrate!
The picture below shows a leading tower paint from the 1980s, which contained all three essential elements of a great tower coating: 1: A shielding, labyrinth-effect platelet-shaped pigment (leafing aluminum), zinc-dust, and corrosion inhibitors appropriate for that time. The resin system consisted of a modified linseed oil, which has been a common ingredient providing maximum wetting and penetration of rusty substrates.
With pressure to remove the leads from coatings in the 1970s came further changes. A major advancement was the use of micaceous iron oxide as a partial replacement for lead pigments. These versions through the 70s and 80s also often contained barium compounds, aluminum, and zinc dust. The various shapes and sizes of these pigments created an excellent barrier coating against the elements. The latest addition to this great coating has been Induron’s addition of ceramic microspheres, which helps increase the WFT/DFT on sharp angles and edges.
So you can see that historically great tower paint has never been all about just one ingredient, but rather the combination of functional pigments in a carefully modified resin system that has created the ideal tower coating. Remember that these coatings rely on the thickness and barrier properties over time to protect the substrate, and are not now, and have never been, “zinc-rich” coatings, which provide galvanic protection to protect the metal substrate, with zinc loadings far above those effective for surface-tolerance over rusted galvanized steel.
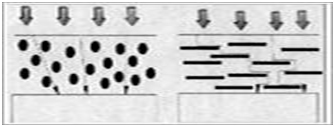
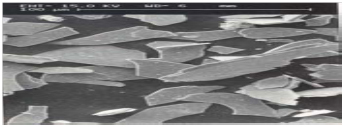
Example of Micaceous Iron Oxide providing overlapping layers, or the “Labyrinth Effect” to slow down the ingress of rain, salt fog, or atmospheric pollutants.
Close-up of the shape of Micaceous Iron Oxide
Properties of Micaceous Iron Oxide vs. Zinc Dust in Barrier Coatings
- MIO=Lamellar formation= Better barrier properties than zinc dust. (The combination better fills the entire resin and forms an effective barrier)
- Spherical fillers do not provide barrier to the coating, whereas MIO= Lamellar particles are an excellent example of shielding/barrier properties.
- MIO=Labyrinth Effect slows down the ingress of water, oxygen and ions.
- MIO=More chemically inert – more chemically resistant than zinc dust.
- Zinc dust is much more reactive to acids and alkalis because if its high and low Ph
- MIO=Heat stable up to its melting point of over 1,000° C (2,700° F).
- MIO is Non-Toxic, Non-Oxidizing, Non-Corrosive & Non-Flammable.
- Ultraviolet (UV) light absorption means faster drying and less TOW.
- MIO flakes are strong UV light absorbers and are very weather resistant.
- MIO=Film reinforcement= strengthens film, making it more durable to last longer.
- MIO=Erosion rates and chalking are greatly reduced when MIO is present.
- MIO=Increased intercoat adhesion AND provides excellent bond to galvanizing.
- MIO=Improved resistance to blistering and increased substrate adhesion.
World’s favorite barrier pigment in coatings for over 100 years! (NOT something new)